NIOSH Publication for Surveillance and Screening of Workers Exposed to Nanoparticles
 Full copy of the publication can be found HERE
Full copy of the publication can be found HERE
“Concerns have been raised about whether workers exposed to engineered nanoparticles are at increased risk of adverse health effects. The current body of evidence about the possible health risks of occupational exposure to engineered nanoparticles is quite small. While there is increasing evidence to indicate that exposure to some engineered nanoparticles can cause adverse health effects in laboratory animals, no health studies of workers exposed to the few engineered nanoparticles tested in animals have been published. The purpose of this document from the National Institute for Occupational Safety and Health (NIOSH) is to provide interim guidance about whether specific medical screening, including performing medical tests on asymptomatic workers, is appropriate for these workers.
Medical screening is only one part of what should be considered a complete safety and health management program. An ideal safety and health management program follows a hierarchy of controls and involves various occupational health surveillance measures. Since specific medical screening of asymptomatic workers exposed to engineered nanoparticles has not been extensively discussed in the scientific literature, this document makes recommendations based upon what is known until more rigorous research can be performed.
Currently there is insufficient scientific and medical evidence to recommend the specific medical screening of workers potentially exposed to engineered nanoparticles. Nonetheless, this lack of evidence does not preclude specific medical screening by employers interested in taking precautions beyond existing industrial hygiene measures. If nanoparticles are composed of a chemical or bulk material for which medical screening recommendations exist, these same screening recommendations would be applicable for workers exposed to engineered nanoparticles as well.
As research into the hazards of engineered nanoparticles continues, vigilant reassessment of available data is critical to determine whether specific medical screening is warranted for workers. In the interim, the following recommendations are provided for workplaces where workers may be exposed to engineered nanoparticles in the course of their work:
* Take prudent measures to control exposures to engineered nanoparticles.
* Conduct hazard surveillance as the basis for implementing controls.
* Continue use of established medical surveillance approaches.
NIOSH will continue to collect and evaluate new research findings and update its recommendations about medical screening programs for workers exposed to nanoparticles. NIOSH will also continue to consider the strengths and weaknesses of establishing exposure registries for workers potentially exposed to engineered nanoparticles for future health surveillance and epidemiological studies.”

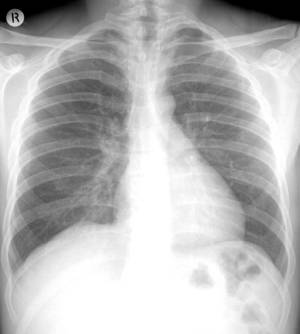 The mortality benefits of restricting the use of asbestos should begin to appear after 2010, when deaths from malignant mesothelioma are expected to peak, according to a report from the CDC.
The mortality benefits of restricting the use of asbestos should begin to appear after 2010, when deaths from malignant mesothelioma are expected to peak, according to a report from the CDC. “Speaking on April 19 at the Experimental Biology 2009 meeting in New Orleans, Dr. Kristine Krajnak, a team leader in the Engineering and Control Technologies Branch of the Health Effects Laboratory Division of NIOSH in Morgantown, West Virginia, describes results from the first study to directly link the different physical responses of tissue that occur with exposure to different vibration frequencies with biological mechanisms underlying the development of vascular dysfunction. Her presentation is part of the scientific program of The American Physiological Society.
“Speaking on April 19 at the Experimental Biology 2009 meeting in New Orleans, Dr. Kristine Krajnak, a team leader in the Engineering and Control Technologies Branch of the Health Effects Laboratory Division of NIOSH in Morgantown, West Virginia, describes results from the first study to directly link the different physical responses of tissue that occur with exposure to different vibration frequencies with biological mechanisms underlying the development of vascular dysfunction. Her presentation is part of the scientific program of The American Physiological Society. “This document reviews what is currently known about nanoparticle toxicity, process emissions and exposure assessment, engineering controls, and personal protective equipment. This updated version of the document incorporates some of the latest results of NIOSH research, but it is only a starting point. The document serves a dual purpose: it is a summary of NIOSH’s current thinking and interim recommendations; and it is a request from NIOSH to occupational safety and health practitioners, researchers, product innovators and manufacturers, employers, workers, interest group members, and the general public to exchange information that will ensure that no worker suffers material impairment of safety or health as nanotechnology develops.”
“This document reviews what is currently known about nanoparticle toxicity, process emissions and exposure assessment, engineering controls, and personal protective equipment. This updated version of the document incorporates some of the latest results of NIOSH research, but it is only a starting point. The document serves a dual purpose: it is a summary of NIOSH’s current thinking and interim recommendations; and it is a request from NIOSH to occupational safety and health practitioners, researchers, product innovators and manufacturers, employers, workers, interest group members, and the general public to exchange information that will ensure that no worker suffers material impairment of safety or health as nanotechnology develops.” “Research indicates that an appropriately designed and maintained upper-room UVGI system may kill or inactivate airborne TB bacteria and increase the protection afforded to healthcare workers while maintaining a safe level of UVGI in the occupied lower portion of the room. The purpose of this document is to examine the different parameters necessary for an effective upper-room UVGI system and to provide guidelines to healthcare managers, facility designers, engineers, and industrial hygienists on the parameters necessary to install and maintain an effective upper-room UVGI system. These guidelines are consistent with previous CDC healthcare guidelines and expand upon them. This document provides an overview of the current knowledge concerning upper-room UVGI systems and research needs. Information from CDC/NIOSH-funded laboratory studies and other relevant studies is combined in this report to provide guidelines for the installation and use of upper-room UVGI systems. Although other pathogenic microorganisms may be killed or inactivated by upper-room UVGI systems, the guidelines were developed for the installation and use of upper-room UVGI systems capable of killing or inactivating surrogates of mycobacteria.”
“Research indicates that an appropriately designed and maintained upper-room UVGI system may kill or inactivate airborne TB bacteria and increase the protection afforded to healthcare workers while maintaining a safe level of UVGI in the occupied lower portion of the room. The purpose of this document is to examine the different parameters necessary for an effective upper-room UVGI system and to provide guidelines to healthcare managers, facility designers, engineers, and industrial hygienists on the parameters necessary to install and maintain an effective upper-room UVGI system. These guidelines are consistent with previous CDC healthcare guidelines and expand upon them. This document provides an overview of the current knowledge concerning upper-room UVGI systems and research needs. Information from CDC/NIOSH-funded laboratory studies and other relevant studies is combined in this report to provide guidelines for the installation and use of upper-room UVGI systems. Although other pathogenic microorganisms may be killed or inactivated by upper-room UVGI systems, the guidelines were developed for the installation and use of upper-room UVGI systems capable of killing or inactivating surrogates of mycobacteria.”