EPA Publishes Guidelines for Methamphetamine Lab Cleanup
 Download HERE:
Download HERE:
 FlammablesPPT.ppt (3.6 MiB, 2,958 hits)
FlammablesPPT.ppt (3.6 MiB, 2,958 hits)
The Voluntary Guidelines for Methamphetamine Laboratory Cleanup provide guidance for individuals responsible for methamphetamine (meth) lab cleanup. The Guidelines are based on an extensive review of the best available science and practices and addresses general cleanup activities, identifies best practices for specific items or materials, discusses sampling procedures, and provides additional technical resources.
Guidelines Questions and Answers:
Why is EPA publishing these voluntary guidelines?
The Methamphetamine Remediation Research Act of 2007 required EPA to develop guidelines for remediating former methamphetamine labs. This document provides those guidelines for States and local agencies to improve “our national understanding of identifying the point at which former methamphetamine laboratories become clean enough to inhabit again.” The legislation also required that EPA periodically update the guidelines, as appropriate, to reflect the best available knowledge and research.
Who should use these guidelines?
The guidelines are geared towards state and local government personnel charged with remediating or otherwise addressing former methamphetamine (meth) labs. This document helps disseminate the best available knowledge and research on meth lab remediation and will also prove useful to cleanup contractors and could be a resource for homeowners.
Does this document create new regulations for meth lab cleanup?
EPA prepared this document based on best current practices to provide voluntary cleanup guidelines to state and local governments, cleanup contractors, industrial hygienists, policy makers and others involved in meth lab remediation. It does not set requirements, but rather suggests a way of approaching meth lab remediation. Those using this document should also consult their appropriate municipal, county or state guidance documents, regulations and statutes. This document is not meant to supersede municipal, county or state guidance documents, regulations or statutes (however this document may be useful as they develop and/or review and revise their own guidelines).

 The Occupational Safety and Health Administration (OSHA) has published the document
The Occupational Safety and Health Administration (OSHA) has published the document  “This document reviews what is currently known about nanoparticle toxicity, process emissions and exposure assessment, engineering controls, and personal protective equipment. This updated version of the document incorporates some of the latest results of NIOSH research, but it is only a starting point. The document serves a dual purpose: it is a summary of NIOSH’s current thinking and interim recommendations; and it is a request from NIOSH to occupational safety and health practitioners, researchers, product innovators and manufacturers, employers, workers, interest group members, and the general public to exchange information that will ensure that no worker suffers material impairment of safety or health as nanotechnology develops.”
“This document reviews what is currently known about nanoparticle toxicity, process emissions and exposure assessment, engineering controls, and personal protective equipment. This updated version of the document incorporates some of the latest results of NIOSH research, but it is only a starting point. The document serves a dual purpose: it is a summary of NIOSH’s current thinking and interim recommendations; and it is a request from NIOSH to occupational safety and health practitioners, researchers, product innovators and manufacturers, employers, workers, interest group members, and the general public to exchange information that will ensure that no worker suffers material impairment of safety or health as nanotechnology develops.” “Research indicates that an appropriately designed and maintained upper-room UVGI system may kill or inactivate airborne TB bacteria and increase the protection afforded to healthcare workers while maintaining a safe level of UVGI in the occupied lower portion of the room. The purpose of this document is to examine the different parameters necessary for an effective upper-room UVGI system and to provide guidelines to healthcare managers, facility designers, engineers, and industrial hygienists on the parameters necessary to install and maintain an effective upper-room UVGI system. These guidelines are consistent with previous CDC healthcare guidelines and expand upon them. This document provides an overview of the current knowledge concerning upper-room UVGI systems and research needs. Information from CDC/NIOSH-funded laboratory studies and other relevant studies is combined in this report to provide guidelines for the installation and use of upper-room UVGI systems. Although other pathogenic microorganisms may be killed or inactivated by upper-room UVGI systems, the guidelines were developed for the installation and use of upper-room UVGI systems capable of killing or inactivating surrogates of mycobacteria.”
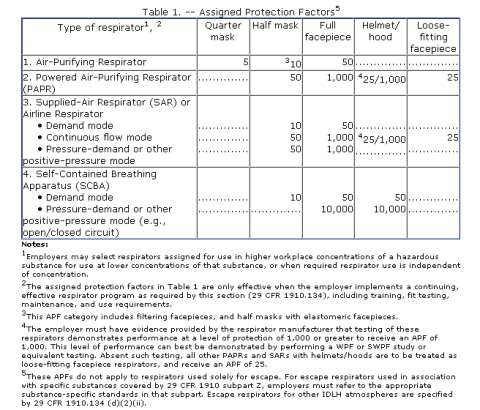
“Research indicates that an appropriately designed and maintained upper-room UVGI system may kill or inactivate airborne TB bacteria and increase the protection afforded to healthcare workers while maintaining a safe level of UVGI in the occupied lower portion of the room. The purpose of this document is to examine the different parameters necessary for an effective upper-room UVGI system and to provide guidelines to healthcare managers, facility designers, engineers, and industrial hygienists on the parameters necessary to install and maintain an effective upper-room UVGI system. These guidelines are consistent with previous CDC healthcare guidelines and expand upon them. This document provides an overview of the current knowledge concerning upper-room UVGI systems and research needs. Information from CDC/NIOSH-funded laboratory studies and other relevant studies is combined in this report to provide guidelines for the installation and use of upper-room UVGI systems. Although other pathogenic microorganisms may be killed or inactivated by upper-room UVGI systems, the guidelines were developed for the installation and use of upper-room UVGI systems capable of killing or inactivating surrogates of mycobacteria.” OSHA has issued a new guidance document for employers who may need to establish and implement a respiratory protection program due to potential exposures to contaminants in workplace air. The document focusues on the mandatory selection provisions of the assigned protection factors (APFs), maximum use concentrations (MUCs) and the use of the APF Table 1 of
OSHA has issued a new guidance document for employers who may need to establish and implement a respiratory protection program due to potential exposures to contaminants in workplace air. The document focusues on the mandatory selection provisions of the assigned protection factors (APFs), maximum use concentrations (MUCs) and the use of the APF Table 1 of