Field Blanks: Why and When to Collect
Quality Assurance in Industrial Hygiene Sampling
Sound industrial hygiene sampling practice requires good quality assurance (QA) practices. QA is defined as:
the maintenance of a desired level of quality in a service or product, especially by means of attention to every stage of the process of delivery or production.
In essence, QA seeks to minimize error and at the same time evaluate sampling performance. Key QA practices include the following:
- use of standard operating procedures (SOPs) for sample collection and analysis;
- use of chain-of-custody and sample-identification procedures;
- instrument standardization, calibration, and verification;
- technician and analyst training;
- sample preservation, handling, and decontamination; and
- use of quality control (QC) samples such as field and transport blanks, duplicates, etc.
Quality Control: Why collect Field Blanks?
One of the methods of quality control in industrial hygiene sampling is through the collection and analysis of “control samples”. Control samples are typically in the form of blanks, duplicates, spiked samples, or split samples. Blanks can be further refined into several categories including: field blanks, transport blanks & media blanks.
- Field Blanks – determine if there was contamination during the collection stage
- Transport Blanks – determine if there was contamination during the shipping stage
- Media Blanks – determine if the media itself is contaminated
Therefore, if quality assurance is our goal, and the use of field blanks (i.e. quality control) are one of our methods for ensuring QA.
How to Collect a Field Blank
Field blanks are clean sampling media and are handled in exactly he same manner as the samples you are collecting, except:
- No air is drawn through them (for air samples), or no surface is wiped with them (for surface / wipe samples),
- They are opened and closed quickly in the sampling area and then resealed, and
- Accompany the actual samples through every stage of the sampling process.
How Many Field Blanks Should I Collect?
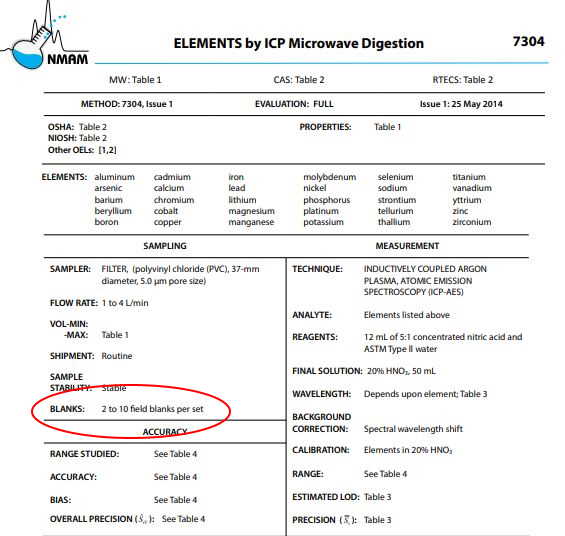
The number of field blanks you should collect should be based on a few considerations (in order of importance), including: sample method, reason for sampling, and budget considerations. The sampling method (e.g. NIOSH or OSHA) should dictate how many field blanks you should collect (see image below), based on the number of samples you collect. Typically, the recommended practice is 10% of your number of samples.
What do I do if the Contaminant is Detected in the Field Blank?
If a contaminant is found on the field blank, the field blank contaminant is typically going to be reported as a mass, and not a concentration (remember, no air volume was drawn through the field blank sample). The mass of contaminant found on the field blank will be subtracted from that found on the actual sample before dividing by the air volume to determine the mass concentration of the contaminant. Additionally, it’s important to note the sampling methods often have a permissible limit of contaminant on the field blank. If this level is exceeded, the samples should be discarded and sampling repeated.
If we do not account for the field blank contamination, the industrial hygienist runs the risk of the reported results being biased high, which can lead to: additional sampling or the implementation of potentially unnecessary controls.
Why Don’t Industrial Hygienists Take Field Blanks?
Practicing industrial hygienists don’t take field blanks for several reasons, including: budgets (10% extra costs for “worthless” blanks can seem excessive), improper training, unfamiliarity with the sampling methodology, and they don’t want competence questioned. However, none of these reasons justify forgoing good QA/QC practices to ensure the integrity of your samples.